Navi Medical Technologies
Neonav®
2024
Concept, Development, Electronics, Production + Packaging
Navi NeoNav
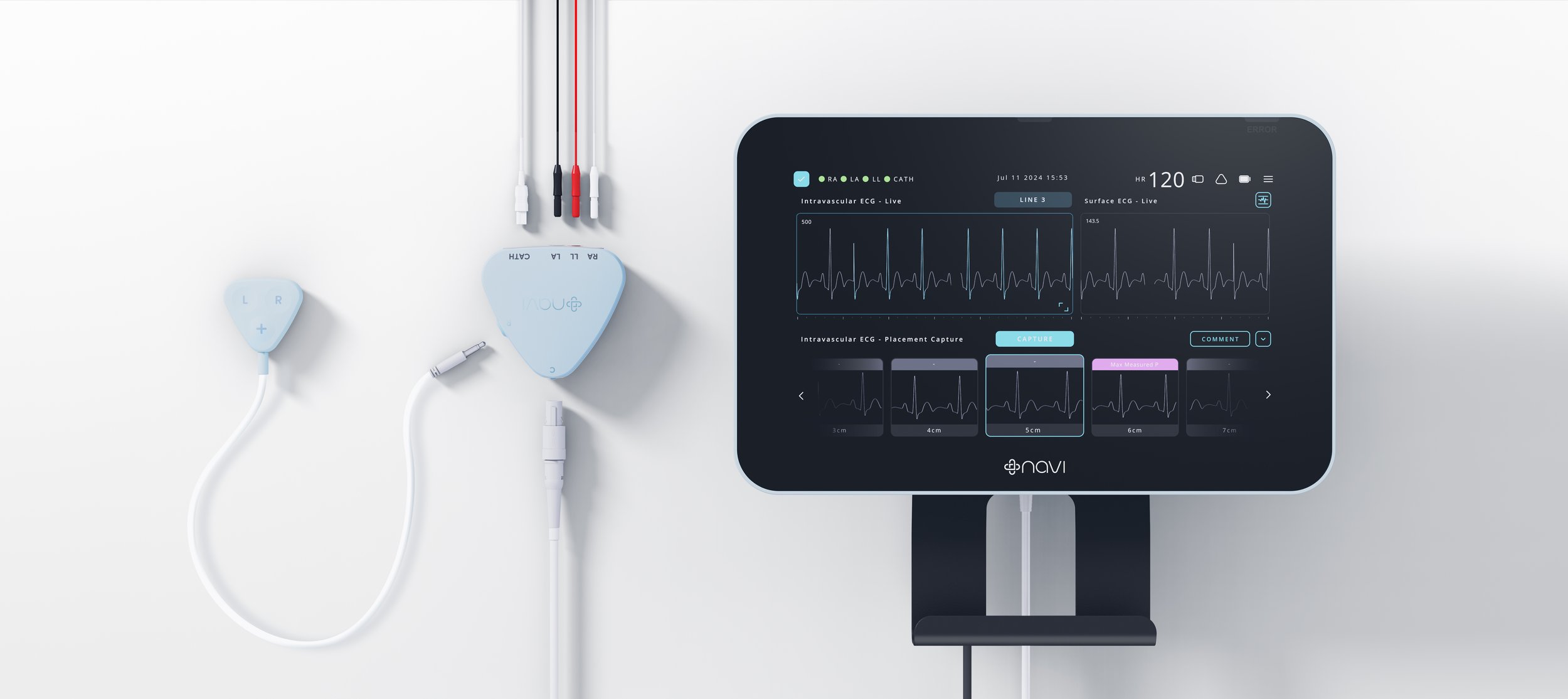
Neonav® is designed to save lives by providing accurate, real-time catheter tip location for critically ill newborns and paediatric patients.
The Neonav® ECG Tip Location System addresses a critical challenge in neonatal and paediatric intensive care units: accurately and safely placing central catheters in the veins of critically ill newborns and children to administer essential medicines, nutrients or fluids. Currently, almost half of these procedures result in catheter misplacement, which, if undetected, can lead to life-threatening complications. This high misplacement rate occurs because clinicians rely on x-rays to confirm catheter location, which can only be done after the procedure. The core need was for a non-invasive system that provides clinicians with immediate, real-time feedback on catheter tip location during and after insertion to improve first-time placement accuracy and reduce complications.
Impact Series © 2024 Breakthrough Victoria. All rights reserved.
Collaborative Development
Multidisciplinary team and extensive research ensures safety, efficacy and optimised usability.
Over an eight-year journey, Navi Medical Technologies has gathered a multidisciplinary team of industry experts, biomedical engineers, partners, and a senior neonatologist to develop and commercialise the Neonav® system. The process involved extensive research in neonatal and paediatric intensive care units across Australia and the USA to gather insights from clinical staff about the difficulties of reliable and repeatable catheter tip location. Several prototype iterations were developed, incorporating feedback from healthcare professionals and undergoing rigorous testing in controlled clinical environments on over 100 patients. This user-focused, quality-driven process ensured the device met safety and efficacy standards, resulting in a user-friendly, non-invasive device that integrates seamlessly into clinical workflows. Its real-time feedback and easy-to-read interface allow clinicians to use it effectively with minimal training.
Innovative Solution
ISO 13485:2016 Compliant Development: Innovative hardware and software integration for real-time feedback.
The Neonav® system's innovative approach includes proprietary software and hardware that deliver accurate ECG signal interpretation, providing real-time guidance to clinicians as they insert and position a tiny catheter near the heart. The system also offers continuous monitoring to detect if a catheter has moved or migrated to an unsafe location over time. Design + Industry (D+I) led the ISO 13485:2016 compliant design and development process, managing multiple interdependencies across industrial design, mechanical engineering, electronics design, UI/UX, firmware/software, including integration of Navi’s proprietary algorithms, and transfer into production. This comprehensive approach ensured the device met the highest standards of safety, reliability, usability, and manufacturing, aligning with Class II Medical Device requirements for regulatory submissions in the United States, Europe, and Australia. Support from Victorian programs and grants like VMRAF, MMGP, TAIP, and MMCP was instrumental in the system's commercialisation.
Proven Impact
A User-Friendly Breakthrough in Pediatric Care with High Accuracy and Accessibility
Extensive research, including the “ECHO” study, sought to compare the Neonav® system's accuracy with the gold-standard technology, ultrasound technology. This study has shown promising results, showing the Neonav having a high agreement rate with Ultrasound. Unlike ultrasound, which requires extensive training and 24/7 access to qualified operators, the user-friendly design of Neonav® reduces the learning curve for clinicians, making it easier to implement across various healthcare settings. The Neonav® system received FDA Breakthrough Device Designation, validating its significant improvement over existing solutions, and potential to have a global impact by improving the standard of care for our youngest and most vulnerable patients.
Medical Device Design + Development: Industrial Design, Mechanical Engineering, Electronics Design, Management into Production
Disclaimer:
The Neonav is currently under development and has not yet been cleared or approved by the U.S. Food and Drug Administration (FDA), Australian Therapeutic Goods Administration (TGA), or any other regulatory authority. All images and videos are intended for illustrative and educational purposes only. The Neonav is not available for commercial sale. The information provided herein is for informational purposes only and should not be construed as a promotional claim regarding the safety, efficacy, or intended use of the device. Any statements regarding potential benefits, uses, or features of the Neonav device are based on preliminary research and should not be interpreted as an endorsement by the FDA or TGA.
The Neonav is not available for sale or use in the United States or Australia until the necessary regulatory approvals and clearances have been obtained. We are committed to complying with all FDA and TGA requirements and will update our communications accordingly as we progress through the regulatory process.